A Chinese COVID-19 candidate vaccine has passed phase one and two clinical trials. The vaccine showed no "serious adverse reactions" with all volunteers generating antibodies after two doses in 28 days, its developer China National Biotec Group (CNBG) said on Tuesday.
The vaccine, developed by a CNBG subsidiary, Wuhan Institute of Biological Products, is the world's first inactivated COVID-19 vaccine.
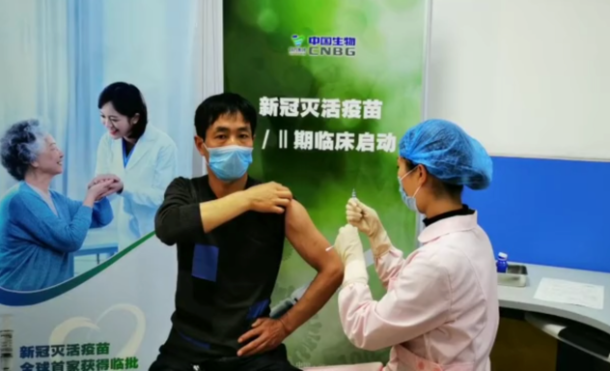
A volunteer in central China's Henan Province is being injected with the inactivated COVID-19 vaccine. /Photo courtesy of CNBG
The human trials began in Wuzhi County, central China's Henan Province on April 12. A total of 1,120 volunteers aged between 18 and 59 were inoculated with two doses in 28 days, according to CNBG.
In the randomized, double-blind and placebo-controlled trial, all volunteers generated high levels of neutralizing antibodies, which can block pathogens from infecting human cells, the company said.
The study is the world's first clinical trial to obtain safety and effectiveness data of a two-dose inactivated COVID-19 vaccine, CNBG said, adding that the clinical trial has lasted the longest period of time (66 days) and got the most comprehensive data, as well as the most satisfying results among all COVID-19 vaccines to date.
The results have provided scientific and evaluable data for COVID-19 prevention and vaccine emergency use in China, the company said.
China has the world's highest number of vaccine candidates for COVID-19, with five undergoing clinical trials.
(CGTN's Liu Yang also contributed to the story.)




